EmTechMIT, Cambridge, MA: In recent years, enterprise R&D in biotechnology has been fundamentally reshaped by the rise of artificial intelligence. At EmTechMIT, Chief Business & Legal Officer Sarah Korman of Isomorphic Labs (ISO), the Alphabet-backed spinout from Google’s DeepMind, offered a behind-the-scenes look at how AI is accelerating the design and development of novel medicines, and why this convergence is poised to redefine what it means to be a drug company.
From AlphaFold to ISO: An AI Heritage
Isomorphic Labs was born in 2021 out of breakthroughs in DeepMind’s AlphaFold, the AI system that revolutionized protein structure prediction. As Korman explained, “We were inspired by the impact AlphaFold 2 had on the industry. Demis [Hassabis] felt strongly at that time that AI was ready to be applied to real-world problems.” The success of AlphaFold provided proof that machine learning could solve deep scientific mysteries, prompting ISO to set an audacious goal: “To one day solve all disease.”
Now four years old, ISO is scaling up from its London headquarters (and new offices in Switzerland and Boston’s Kendall Square), with over 300 interdisciplinary staff. “We are a drug discovery company, meaning that we’re advancing our own therapeutics,” said Korman. Their internal pipeline focuses on oncology and immunology, fields where new drugs could radically improve patient outcomes, and includes partnerships with industry giants like Eli Lilly and Novartis. Notably, the Novartis collaboration doubled in scope in 2025, signaling strong faith in AI-driven medicinal design.
The “AI-First” Organization
ISO distinguishes itself as an “AI-first company”, not just applying machine learning on top of legacy drug pipelines, but building foundational systems that can generalize across disease classes, therapeutic areas, and drug modalities. “From the very beginning, we have developed systems and approaches applicable across disease classes. We can advance drug candidates in any TA [therapeutic area] without fine-tuning our engine,” Korman emphasized.

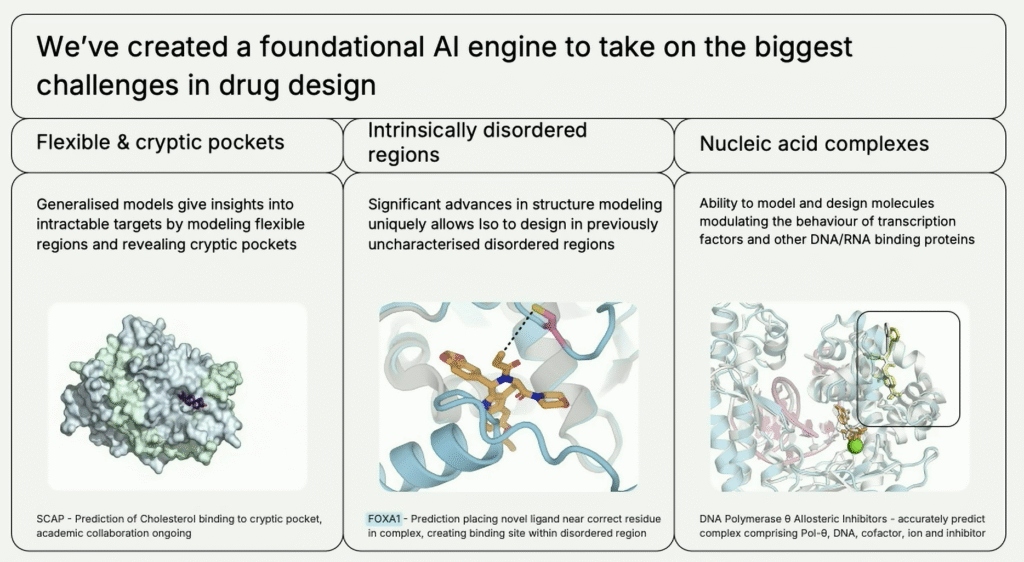
In effect, the company engineers AI models capable of atomic-level simulation and binds that with massive computational power (“planet scale compute”) and proprietary datasets generated specifically for model needs. The result: a unified foundational drug design engine comprising “at least 5 or 6 AlphaFold-level advancements” capable of handling everything from protein folding to molecule binding affinities, dynamic structure modeling, toxicology prediction, and beyond.
Crucially, the platform isn’t a black box. Drug hunters interface directly with ISO’s engine through user-friendly software, receiving near real-time predictions that inform the next steps in candidate selection or synthesis. “They can work with this on the train, at home with these models, offering real-time or near real-time predictions,” Korman shared.
Redefining Productivity and Collaboration
ISO’s approach yields extraordinary gains in productivity. “We can assess billions of hypotheses per week,” Korman said, “and we can get near 100% hit rates on 200 or less molecules, dramatically lower than in traditional approaches.” Because so much candidate selection is performed in silico, wet lab synthesis is outsourced to a network of contract research organizations (CROs), allowing ISO to remain agile and capital-efficient.
This silico-first model also sets ISO apart culturally. “We wouldn’t put ourselves in either camp”, neither pure technology company, nor classic drug maker, Korman asserted. Blending tech and biotech cultures requires intention, especially as “the tech cycle moves rapidly, but the drug life cycle moves slowly.” The challenge: balancing software’s quick iteration with the patience required for rigorous clinical progress and patent protection in pharma.
ISO’s interdisciplinary mindset is supported at every level. “We live this every day. It’s not only in how we practice with each other, it’s a mindset, it guides everything we do.” Drug hunters and ML engineers sit side-by-side; leadership is accessible; communication is prioritized. Korman cited Demis Hassabis as both a visionary and hands-on collaborator: “Demis is there, he sits out in the open with all of us and he truly means what he says.”
Data Strategy: Beyond Open Source
Open source models such as AlphaFold have democratized predictive protein science; ISO, however, maintains its edge by curating proprietary and synthetic datasets, supplementing with in-licensed data and generating specialized knowledge to lift model performance. “We’ve been generating our own datasets since the very start of the company, specific to our model’s needs. We have a data team that sits with our drug designers and ML engineers; they know down to the volume and type the exact quantity of data that we need.”
Experimentation hasn’t become obsolete, despite the explosion of data and in silico modeling. “We know AlphaFold is not perfect, so definitely there’s room to be experimenting to continue to optimize these protein predictions. We still supplement our data.” The balance between computational prediction and wet lab validation remains crucial, especially as ISO moves toward preclinical and eventually clinical testing.
What Kind of Drugs, and Who Is the Inventor?
ISO’s engine is designed to produce drugs across modalities: small molecules, biologics, peptides, and molecular glues. Its initial focus is on oncology and immunology, but partnerships have spanned modalities. “It’s all atoms,” Korman quipped, “so it produces drug candidates across the board.”
One provocative question was whether an AI could be named as the inventor of a new drug. “You need a human named as an inventor,” Korman clarified, citing US patent office rules and emergent case law. “I don’t know of an agent yet that can generate drugs without any type of human interaction.” Patent attribution is still catching up to AI-augmented invention, but for now, the human engineer remains the legally-recognized creator.
Competitive Edge and Ecosystem Thinking
In a landscape rapidly expanding with open source and industry challengers (including MIT’s own BOLT protein modeling team), ISO’s competitive advantage lies in its proprietary engine and its organization. “We love seeing advancements in the space. We think this is an ecosystem.” Still, Korman stressed that modeling protein structures is “just one very small piece” of the puzzle; ISO aims to integrate many models, each representing a layer of the drug development process, into one harmonized platform.
A New Kind of Drug Company
Korman closed with an affirmation of ISO’s grand mission and its ethics. She quoted Demis Hassabis: “There’s no more important application for AI than helping improve human health.” The company’s passion is matched by urgency. “Drug discovery is more expensive than ever; patients can’t wait. We feel ready for the first time, things have converged, data, algorithms, people, to really make a difference.”
Isomorphic Labs stands as an emblem for what enterprise R&D can become: not slow, reactive, and siloed, but integrated, rapid, and radically targeted. At the intersection of compute and compassion, Korman and ISO are engineering not just drugs, but the architecture for a future where personalized medicine can arrive at speed.
For more information, please visit the following:
Website: https://www.josephraczynski.com/
Blog: https://JTConsultingMedia.com/
Podcast: https://techsnippetstoday.buzzsprout.com
LinkedIn: https://www.linkedin.com/in/joerazz/


Leave a Reply
You must be logged in to post a comment.